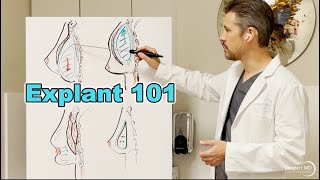
Unfortunately, some women naturally develop conditions that cannot be predicted or prevented. When this is the case, Dr. Lampert finds that revision surgery is usually indicated.
Ruptured Implants
There are two types of implants: those filled with saline and those filled with silicone gel. All implants are contained within a silicone elastomer shell. When saline implants rupture, the deflation is usually immediately obvious due to the saline solution leaking into the chest. Saline solution is safely absorbed into the body so that this is not a health concern. However, any implant, once ruptured, must be removed and replaced, if desired. Sometimes a slow leak leads to a slowly deflating saline implant.
Silicone gel implants have greatly improved over the last 20 years. They rarely spontaneously leak, and rupture rates are very low. Ruptures can occur due to outside forces such as:
- Trauma, as in a car accident
- Mammogram pressure
- Accidental puncture during biopsy or other surgical procedures
The newer cohesive gel implants (or “gummy bear implants”) have a fill that remains in the implant shell after a leak. With silicone implants, it may not be immediately obvious that there is a problem. The FDA recommends that women with silicone gel implants should commit to having an MRI every 3 years after their initial surgery and then every 2 years after that to detect any possible rupture that may be present. Additionally, any silicone gel that escapes the shell is usually contained within the scar capsule naturally formed around the implant.
In the past, there was considerable fear surrounding silicone gel leaking into the body. However, extensive research has shown safety with the use of both silicone and saline breast implants for breast augmentation. An MRI can usually detect an implant rupture, which is the only way that many leaking silicone implants are detected. In the case of a leaking saline implant, the affected breast will appear smaller—usually immediately, unless there is a very slow leak. PIP breast implants, which were never allowed in the US but were popular in France, had a very high incidence of rupture and have since been removed from the market. Patients with these implants should have them removed as soon as possible.